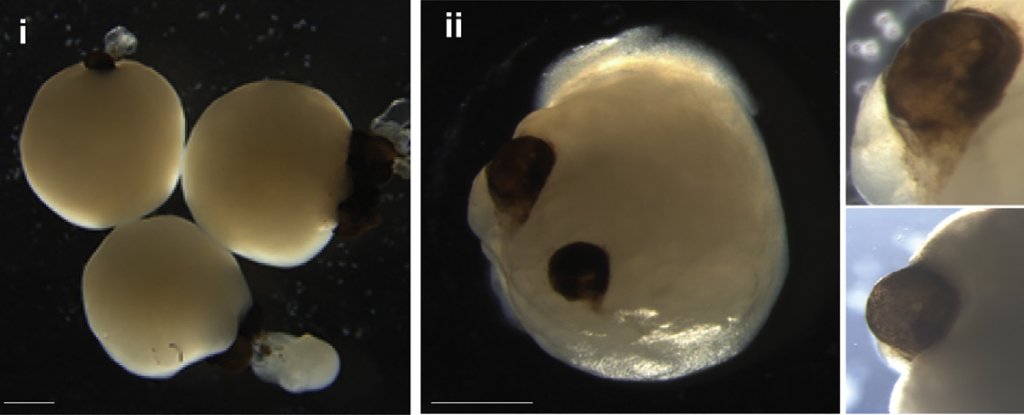
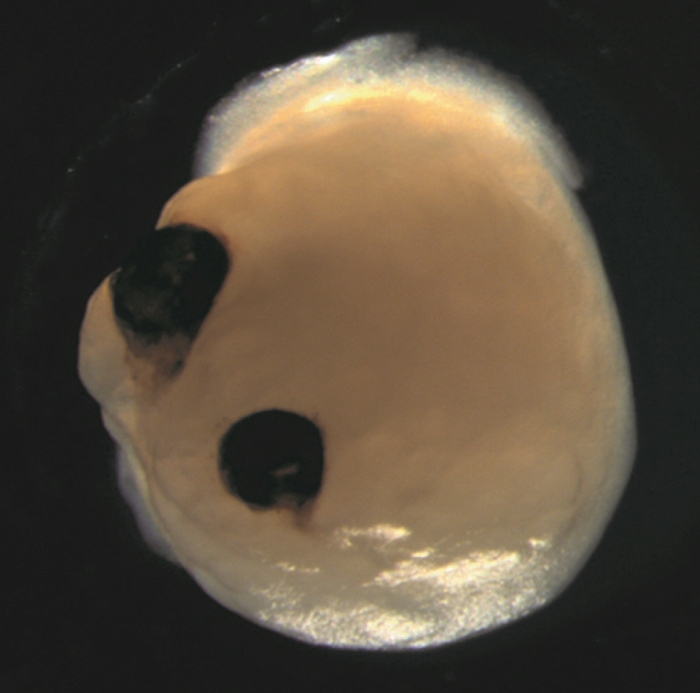
In a breakthrough study from 2021, scientists revealed that ‘mini brains’ cultivated in a lab using stem cells spontaneously developed rudimentary eyes. This fascinating discovery occurred within small human-derived brain organoids grown in laboratory dishes, showcasing the emergence of bilaterally symmetrical optic cups—a process mirroring the formation of eye structures in human embryos.
The significance of this achievement extends to advancing our comprehension of eye differentiation and development, as well as providing insights into various eye diseases.
Neuroscientist Jay Gopalakrishnan from the University Hospital Düsseldorf in Germany expressed enthusiasm, stating, “Our work highlights the remarkable ability of brain organoids to generate primitive sensory structures that are light-sensitive and contain cell types similar to those found in the body.” This breakthrough opens avenues for studying brain-eye interactions during embryo development, modeling congenital retinal disorders, and producing patient-specific retinal cell types for personalized drug testing and transplantation therapies.
Brain organoids, contrary to common perception, aren’t authentic brains in the traditional sense. They are compact, three-dimensional formations cultivated from induced pluripotent stem cells—cells derived from adult humans and reprogrammed into stem cells capable of developing various tissue types.
In this instance, these stem cells are coaxed into forming clusters of brain tissue, lacking attributes such as thoughts, emotions, or consciousness.
These ‘mini brains’ serve vital roles in research, especially where using living brains would be impractical or ethically challenging—applications include drug response testing and observing cell development in specific adverse conditions.
Gopalakrishnan and team, in this particular study, aimed to scrutinize eye development.
While previous research utilized embryonic stem cells to generate optic cups, structures forming nearly the entire eye globe during embryonic development, Gopalakrishnan’s team sought to integrate these structures within brain organoids. This approach offered the advantage of observing the concurrent growth of two distinct tissue types, providing insights beyond the isolated development of optic structures.
In their paper, the researchers emphasized, “Eye development is a complex process, and understanding it could allow underpinning the molecular basis of early retinal diseases.” They highlighted the significance of studying optic vesicles, the eye’s primordium attached to the forebrain, crucial for proper eye formation.
Prior research in organoid development indicated the presence of retinal cells, but optic structures didn’t manifest. To address this, the team adjusted their protocols, avoiding the imposition of purely neural cell development in the early stages. Additionally, they introduced retinol acetate to the culture medium as a facilitator for eye development.
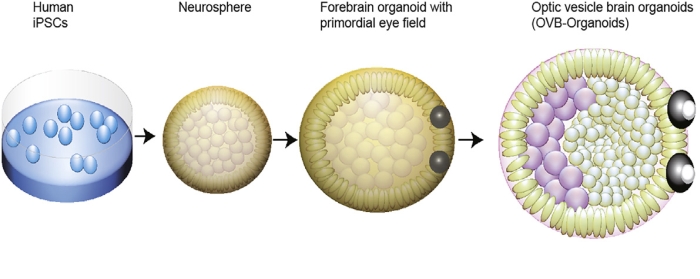
Nurtured with precision, these developing brain organoids showcased the formation of optic cups as early as 30 days into their growth, with clear visibility of the structures by the 50th day. This timeline aligns with the normal eye development in human embryos, making these organoids a valuable tool for delving into the nuances of this intricate process.
The implications extend beyond mere timing. The optic cups housed diverse retinal cell types that organized into responsive neural networks sensitive to light. Notably, they also contained lenses and corneal tissue. Moreover, these structures demonstrated retinal connectivity with regions of the brain tissue.
“In the mammalian brain, nerve fibers of retinal ganglion cells reach out to connect with their brain targets, an aspect that has never before been shown in an in vitro system,” highlighted Gopalakrishnan.
The reproducibility is noteworthy—out of the 314 brain organoids cultivated, 73 percent developed optic cups. The team aspires to devise strategies for maintaining the viability of these structures over longer periods, unlocking vast potential for more in-depth research.
Their paper notes, “Optic vesicle-containing brain organoids displaying highly specialized neuronal cell types can be developed, paving the way to generate personalized organoids and retinal pigment epithelial sheets for transplantation.”
The researchers envision these as next-generation organoids, instrumental in modeling retinopathies stemming from early neurodevelopmental disorders.
This groundbreaking research has been documented in Cell Stem Cell.