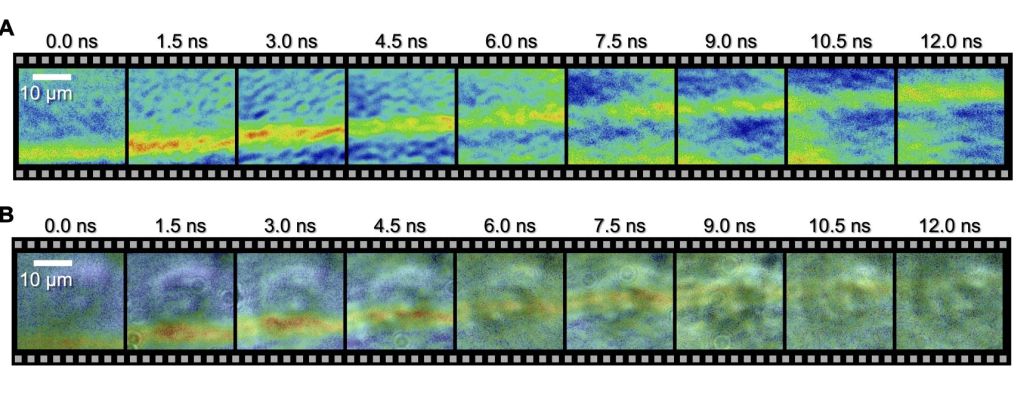
In a remarkable display of innovation, researchers have successfully recorded a minuscule shock wave coursing through an individual human cell – a groundbreaking achievement captured on film for the very first time.
Employing an innovative technique known as a spectrum circuit, a group led by engineer Takao Saiki from the University of Tokyo has accomplished nanoscale precision and the necessary speed to systematically record, frame by frame, the progression of an underwater acoustic shock traveling through a cultured HeLa cell, capturing the entire process from start to finish.
The images produced provide fresh insights into the interaction between shocks and biological cells. This groundbreaking discovery not only sheds light on the dynamics of shockwave interaction but also paves the way for advancements in comprehending shockwave therapy and classifying cells according to their acoustic properties.

For the first time in history, as far as we know, we have directly observed the interaction between a biological cell and a shock wave, and experimentally demonstrated that the velocity of the shock wave propagating inside the cell is faster than the outside of the cell,
Saiti explains.
Furthermore, our approach has enabled us to demonstrate high-speed photography across a wide time range, which includes picosecond (one-trillionth of a second), nanosecond (one-billionth of a second) and millisecond (one-thousandth of a second) timescales.
Capturing images of individual cells poses significant challenges. Their delicate nature makes them susceptible to damage, and their minuscule size demands exceptionally high-resolution capabilities. Furthermore, due to their diminutive scale, any movement within the cell occurs rapidly, making it easy to miss crucial details if you so much as blink.
Any system aiming to capture the propagation of shockwaves through a cell must meet specific criteria. It must operate on extremely minute spatial and temporal scales while ensuring the cell remains undamaged throughout the process.
The spectrum circuit operates as an optical circuit, utilizing light rather than electricity. The team employs this technology to generate laser pulses that are non-damaging to the cell. These pulses are directed into a water-filled dish, interacting with a cell at various nanosecond intervals.
Subsequently, they applied an imaging method known as Sequentially Timed All-Optical Mapping Photography or STAMP. STAMP utilizes burst photography to create a sequence of consecutive images within short timescales.
The camera was employed to capture the shockwave’s journey through the cell, taking snapshots at 1.5 nanosecond intervals, with an exposure time of 44 picoseconds for each frame. The outcomes distinctly depict the progression of the wavefront across the cell.
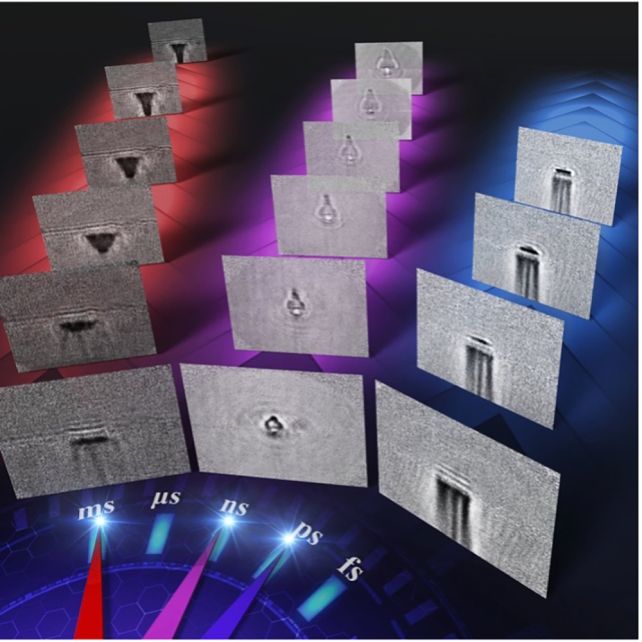
This technology extends beyond biological imaging; the scientists also utilized it to visualize the impacts of laser ablation on glass.
By directing femtosecond (one-quadrillionth of a second) laser pulses at a glass sheet, they could meticulously observe the entire sequence—from the laser’s impact on the glass to the ensuing shockwave and ablation—across various time scales.
According to the team, this technology provides scientists with a fresh tool to comprehend swift microscopic interactions, dissecting them into a series of processes that can contribute to the development of more effective control tools. The implications of this advancement are far-reaching.
Our technology provides opportunities to reveal useful but unknown high-speed phenomena by enabling us to observe and analyze such ultrafast processes,
says engineer Keiichi Nakagawa of the University of Tokyo.
Next, we are planning to use our imaging technique to visualize how cells interact with acoustic waves, like those used in ultrasound and shock wave therapy. By doing this, we aim to understand the primary physical processes that activate subsequent therapeutic effects in the human body.
The findings have been officially documented in Science Advances.