Physicists have achieved a groundbreaking feat by crafting, in a laboratory setting, an unusual and delicate structure called a trilobite Rydberg molecule for the first time.
The creation and observation of these unique atomic formations have provided researchers with fresh perspectives on the quantum behavior of electrons during their interaction near atoms.
Given that their chemical bonds are unlike any others we know, these discoveries provide opportunities for enhancing theoretical models of molecules and gaining insights into their dynamics.
Rydberg molecules originate from a type of atom called a Rydberg atom. In a regular atom, there’s the nucleus surrounded by its tiny swarm of electrons. When a small amount of energy is added to the atom, the electron swarm expands slightly, causing the atom to become a bit larger and more loosely bound.
A Rydberg atom is formed by injecting a substantial amount of energy under conditions that enable it to retain its electrons. It expands significantly, reaching sizes of many microns, and the electrons become as loosely bound as possible without detaching.
Due to their relaxed nature, Rydberg atoms exhibit an exaggerated behavior, rendering them valuable for conducting experiments.
Molecules are configurations of atoms that bond together, whether through electron sharing or disparate charges. Using a Rydberg atom results in a Rydberg molecule, but the way these atoms adhere to each other can differ significantly from the bonds in more traditional molecules.
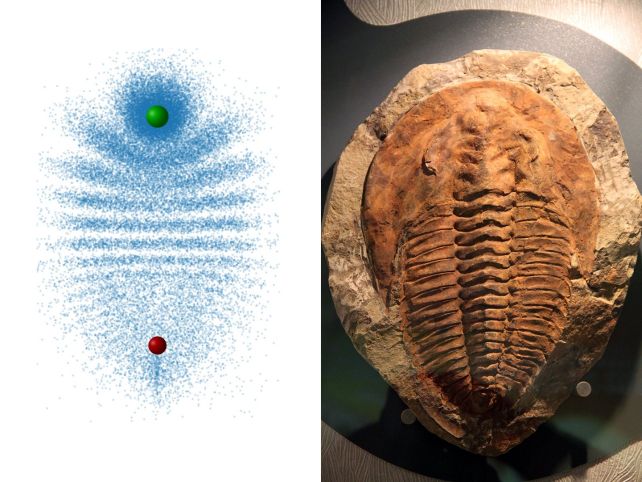
They can exhibit distinct appearances, featuring electron distribution patterns that may resemble, for example, a trilobite or a butterfly.
Physicist Max Althön from the University of Kaiserslautern-Landau, along with a team of scientists in Herwig Ott’s laboratory, has successfully produced, for the first time, entirely pure trilobite Rydberg molecules.
Beginning with rubidium atoms, cooled to an ultra-low temperature of just 0.0001 degrees above absolute zero, the researchers employed a laser to elevate certain atoms into Rydberg states.
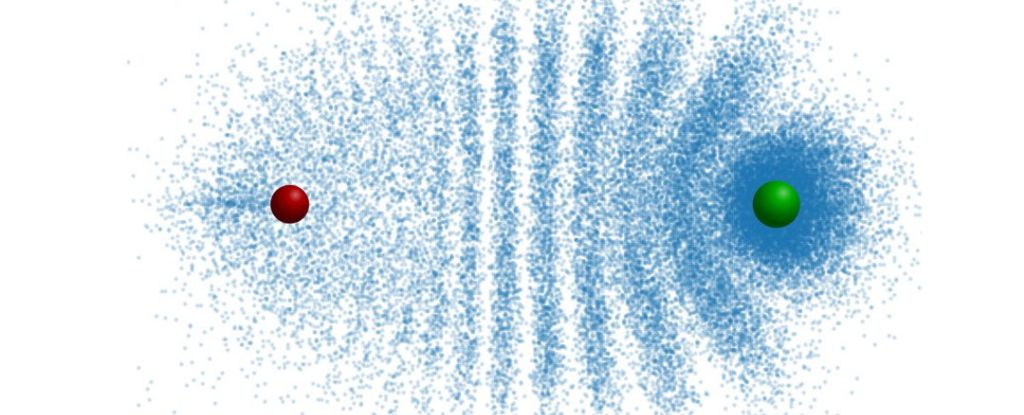
“In this process, the outermost electron in each case is brought into far-away orbits around the atomic body,” Ott says. “The orbital radius of the electron can be more than one micrometer, making the electron cloud larger than a small bacterium.”
A Rydberg molecule is formed by introducing a ground-state atom, one that hasn’t been excited into a Rydberg state, into the expansive electron cloud of the Rydberg atom. The two atoms adhere together not through conventional chemical bonds but through an unusual quantum attraction.
“It is the quantum mechanical scattering of the Rydberg electron from the ground state atom, which sticks the two together,” Althön explains.
“Imagine the electron rapidly orbiting around the nucleus. On each round trip, it collides with the ground state atom. In contrast to our intuition, quantum mechanics teaches us that these collisions lead to an effective attraction between the electron and the ground state atom.”
Due to successive collisions, the electrons arrange themselves into an interference pattern that mimics the segmented carapace of a trilobite.
It possesses some other intriguing and peculiar characteristics. The size of the molecular bond is nearly identical to the dimensions of the Rydberg orbit, which is significant on atomic scales. Additionally, the intensity of the attraction between the electron and the ground-state atom is remarkably strong.
This indicates that Rydberg molecules exhibit a greater electric dipole moment compared to any other molecule. This refers to the separation between positive and negative electric charges, commonly known as polarity.
The trilobite Rydberg molecules observed by Althön and his colleagues possess an electric dipole moment exceeding 1,700 debye, which is exceptionally high. In comparison, water molecules typically have a measure of less than 2 debye.
Physicists now have a new tool for testing and understanding the quantum realm, thanks to the capability not only to create but also to probe pure trilobite Rydberg molecules.
These findings open potential applications in quantum information processing. Moreover, researchers suggest broader applications for studying these unique molecules across various species.
“In conclusion, we have measured two vibrational series of pure trilobite Rydberg molecules by employing three-photon photoassociation,” they write. “With this method, the creation of trilobite molecules in any element that has a negative s-wave scattering length should be possible.”
The study has been published in Nature Communications.