Jellyfish appear quite simple at first glance—gelatinous blobs lacking brains, hearts, or blood, drifting with the ocean currents.
However, simplicity doesn’t equate to a lack of complexity. On the contrary, jellyfish are highly efficient, positioning them as one of the most successful animal groups on the planet.
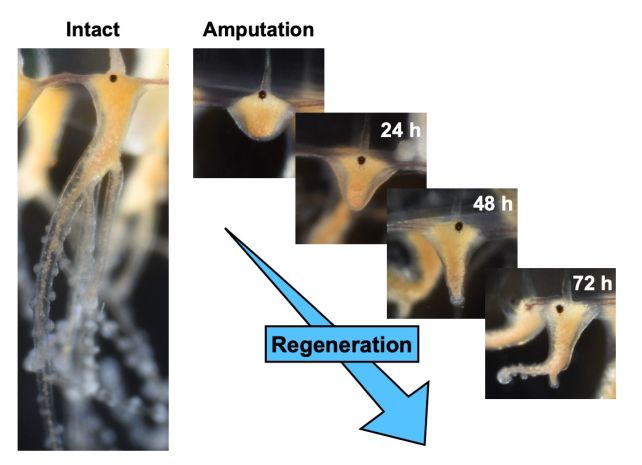
Crucial to their success is their tentacles – lengthy appendages that ensnare their prey, delivering paralyzing toxins to facilitate uninterrupted digestion. If anything disrupts these tentacles, jellyfish have the remarkable ability to swiftly regrow them.
Examining a diminutive jellyfish species known as Cladonema pacificum, a team of scientists led by biologist Sosuke Fujita from the University of Tokyo has uncovered the cellular mechanisms responsible for this remarkable healing ability.
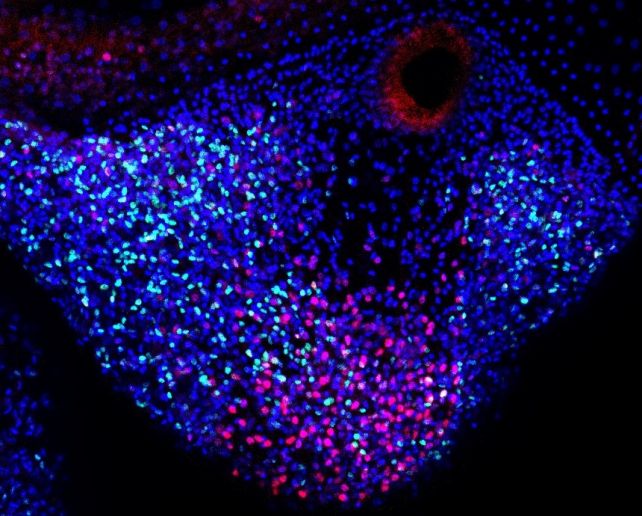
The critical factor for regeneration, observed in various organisms from insects to vertebrates like salamanders, is a cluster of cells known as a blastema. It’s now understood that jellyfish initiate the growth of their blastema, in part, from repair-specific proliferative cells—cells with stem-like properties actively undergoing growth and division but not yet specialized. These cells resemble stem cells as they possess the ability to transform into various cell types as needed.
Importantly, these stem-like proliferative cells in blastema are different from the resident stem cells localized in the tentacle,
says biologist Yuichiro Nakajima of the University of Tokyo. Repair-specific proliferative cells mainly contribute to the epithelium – the thin outer layer – of the newly formed tentacle.
To unravel the mechanisms behind the jellyfish’s regenerative prowess, the scientists meticulously excised tentacles, observed the initiation of the regeneration process, and subsequently euthanized and dissected the creatures. Different stains were employed during the dissection to label distinct cells for analysis.
Jellyfish consistently harbor stem cells within and around their tentacles. These cells, still undifferentiated and without specific functions, possess the ability to develop into any cell type required by the body. They play a crucial role in the continuous maintenance and repair of the jellyfish’s body throughout its lifespan.
Yet, the repair-specific proliferative cells emerge only in response to injury in jellyfish – they are exclusive to the repair and regeneration of injured body parts. This mechanism shares similarities with the repair-specific cells in salamanders, which exhibit bilateral symmetry in their development, in contrast to the distinctive radial development observed in jellyfish.
In this study, our aim was to address the mechanism of blastema formation, using the tentacle of cnidarian jellyfish Cladonema as a regenerative model in non-bilaterians,
Fujita explains.
Given that repair-specific proliferative cells are analogues to the restricted stem cells in bilaterian salamander limbs, we can surmise that blastema formation by repair-specific proliferative cells is a common feature independently acquired for complex organ and appendage regeneration during animal evolution.
Salamanders and jellyfish are starkly distinct from each other; their last common ancestor with bilaterians existed hundreds of millions of years ago, and their evolutionary paths diverged significantly. The discovery of a shared repair mechanism in both is intriguing, hinting at a possible case of convergent evolution, where dissimilar organisms independently develop similar traits.
Currently, we lack the tools to unravel the emergence of repair-specific proliferative cells in jellyfish. According to the researchers, this represents a crucial next step, as understanding this process could pave the way for developing methods to grant humans the ability to regenerate body parts.
“Ultimately,” Nakajima says, understanding blastema formation mechanisms in regenerative animals, including jellyfish, may help us identify cellular and molecular components that improve our own regenerative abilities.
The findings have been documented in PLOS Biology.